Questions
2HCl(aq) + Na2S2O3(aq)--> S(s) + H2SO3(aq) + 2NaCl(aq)
Decreasing the concentration of Na2S2O3(aq) decreases the rate of reaction
because the
(1) activation energy decreases
(2) activation energy increases
(3) frequency of effective collisions decreases
(4) frequency of effective collisions increases
H2(g) + I2(g) + heat--> 2HI(g)
Which change favors the reverse reaction?
(1) decreasing the concentration of HI(g)
(2) decreasing the temperature
(3) increasing the concentration of I2(g)
(4) increasing the pressure
temp can be considered "heat"
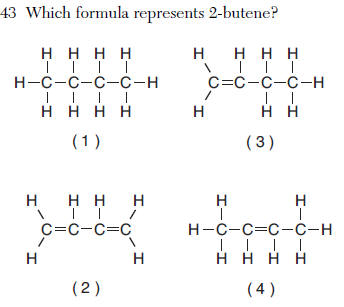
-ene a double bond
2- the double bond is on the second carbon
Fe2O3 + 2Al--> Al2O3 + 2Fe
During this reaction, the oxidation number of Fe changes from
(1) +2 to 0 as electrons are transferred
(2) +2 to 0 as protons are transferred
(3) +3 to 0 as electrons are transferred
(4) +3 to 0 as protons are transferred
(1) Cl2(g) + 2NaBr(aq)-->Br2(l) + 2NaCl(aq)
(2) Cl2(g) + 2NaF(aq)--> F2(g) + 2NaCl(aq)
(3) I2(s) + 2NaBr(aq)--> Br2(l) + 2NaI(aq)
(4) I2(s) + 2NaF(aq)--> F2(g) + 2NaI(aq)
Cl2 can replace Br2
(1) 1.0 L of 2.0 M HCl(aq)
(2) 2.0 L of 2.0 M HCl(aq)
(3) 3.0 L of 0.50 M HCl(aq)
(4) 4.0 L of 0.50 M HCl(aq)
M=mol/Liters
(1) pH 1 to pH 2 (3) pH 2 to pH 1
(2) pH 1 to pH 3 (4) pH 3 to pH 1
100 has 2 zeros pH changes by 2
(1) bromthymol blue (3) litmus
(2) bromcresol green (4) thymol blue
HCl(g) + H2O(l)--> X(aq) + Cl--(aq)
Which ion is represented by X?
(1) hydroxide (3) hypochlorite
(2) hydronium (4) perchlorate
thyroid gland disorders?
(1) carbon-14 (3) cobalt-60
(2) potassium-37 (4) iodine-131