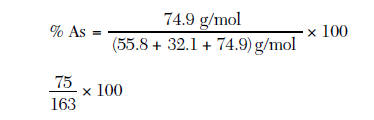Question 83
Arsenic is often obtained by heating the ore arsenopyrite, FeAsS. The decomposition of FeAsS is represented by the balanced equation below. FeAsS(s) ==heat==>FeS(s) + As(g) In the solid phase, arsenic occurs in two forms. One form, yellow arsenic, has a density of 1.97 g/cm3 at STP. The other form, gray arsenic, has a density of 5.78 g/cm3 at STP. When arsenic is heated rapidly in air, arsenic(III) oxide is formed. Although arsenic is toxic, it is needed by the human body in very small amounts. The body of a healthy human adult contains approximately 5 milligrams of arsenic. 83 Calculate the percent composition by mass of arsenic in arsenopyrite. Your response must include both a correct numerical setup and the calculated result. [2] 1 credit for the set up
1 credit for the answer 46.0% |