Questions
(1) CuO (3) Cu2O
(2) CuO2 (4) Cu2O2
2:1 ratio
(1) Ta (3) Te
(2) Tc (4) Ti
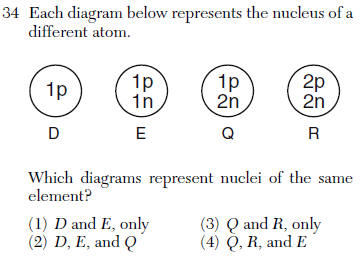
then use Table S to match up the element
(1) aluminum (3) magnesium
(2) argon (4) sodium
group 2
(1) decreases, only
(2) increases, only
(3) decreases, then increases
(4) increases, then decreases
CaO(s) + CO2(g) ==>CaCO3(s) + heat
What is the total mass of CaO(s) that reacts completely with 88 grams of CO2(g) to produce 200. grams of CaCO3(s)?
(1) 56 g (3) 112 g
(2) 88 g (4) 288 g
conservation of mass
(1) CH3 (3) C3H
(2) C2H6 (4) C6H2
2-6
1-3
H2(g) + Cl2(g) ==>2HCl(g) + energy
Which statement describes the energy changes in this reaction?
(1) Energy is absorbed as bonds are formed, only.
(2) Energy is released as bonds are broken, only.
(3) Energy is absorbed as bonds are broken, and energy is released as bonds are formed.
(4) Energy is absorbed as bonds are formed, and energy is released as bonds are broken.
bonds are broken then formed
(1) 0.10 M KCl(aq)
(2) 0.10 M K2SO4(aq)
(3) 0.10 M K3PO4(aq)
(4) 0.10 M KNO3(aq)
KCl-2 K2SO4-3
K3PO4 -4 KNO3 - 2
(1) 1.3 M (3) 3.0 M
(2) 2.0 M (4) 0.75 M
2 moles/1.5L= 1..3M