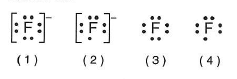Questions
Explanations
31 Which electron shell contains the valence electrons of a radium atom in the ground state?
(1) the sixth shell (2) the second shell
(3) the seventh shell (4) the eighteenth shell
you needed to fill in the 2-8-
32 Each diagram below represents the nucleus of
an atom. How many different elements are represented by the diagrams?

(1) 1 (3) 3
(2) 2 (4) 433 Chlorine and element X have similar chemical properties. An atom of element X could have an electron configuration of
(1) 2-2 (2) 2-8-1 (3) 2-8-8 (4) 2-8-18-7
34 Which group of elements contains a metalloid?
(1) Group 8 (3) Group 16
(2) Group 2 (4) Group 18
35 Which Lewis electron dot diagram represents a
fluoride ion.