Questions
Explanations
26 Given the equation representing a system at equilibrium:
N2(g) + 3H2(g) <==> 2NH3(g)
Which statement describes this reaction at equilibrium?
(1) The concentration of N2(g) decreases.
(2) The concentration of N2(g) is constant.
(3) The rate of the reverse reaction decreases.
(4) The rate of the reverse reaction increases.
or the rates are equal
con con requal
27 The acidity or alkalinity of an unknown aqueous solution is indicated by its
(1) pH value
(2) electronegativity value
(3) percent by mass concentration
(4) percent by volume concentration
28. The laboratory process in which the volume of a solution of known concentration is used to determine the concentration of another solution is called
(1) distillation (2) fermentation
(3) titration (4) transmutation
29 Which list of nuclear emissions is arranged in order from the greatest penetrating power to the least penetrating power?
(1) alpha particle, beta particle, gamma ray
(2) alpha particle, gamma ray, beta particle
(3) gamma ray, alpha particle, beta particle
(4) gamma ray, beta particle, alpha particle
smallest (no mass) to biggest
30 Given the diagram representing a reaction:
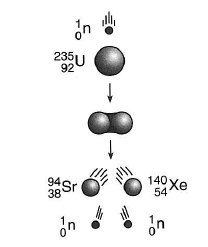
Which type of change is represented?
(1) fission (2) fusion
(3) deposition (4) evaporation