Questions
Explanations
46 A rigid cylinder with a movable piston contains 50.0 liters of a gas at 30.0°C with a pressure of 1.00 atmosphere. What is the volume of the gas in the cylinder at STP?
(1) 5.49 L (3) 55.5 L
(2) 45.0 L (4) 455 L
temp in kelvin
47. Given the potential energy diagram for a chemical reaction:
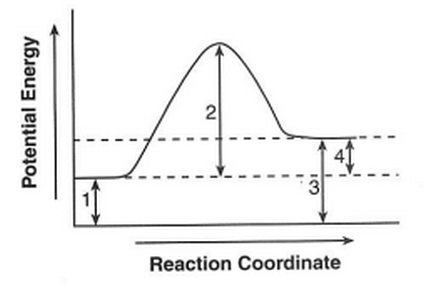
Which numbered interval represents the heat of reaction?
(1) 1 (3) 3
(2) 2 (4) 4
difference between the products and reactants
floating triangle
Base your answers to questions 48 and 49 on the graph below and on your knowledge of chemistry.
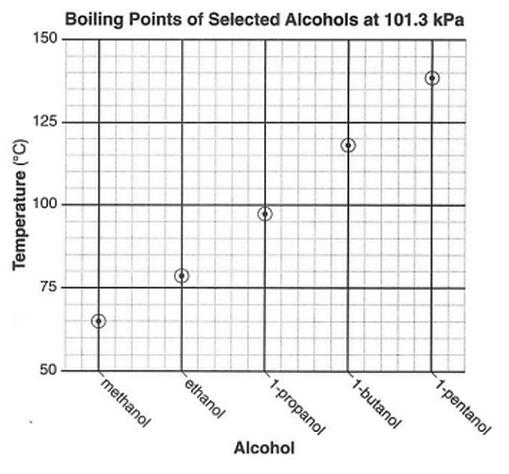
48 What is represented by the number "1" in the IUPAC name for three of these alcohols?
(1) the number of isomers for each alcohol
(2) the number of -OH groups for each carbon atom in each alcohol molecule
(3) the location of an -OH group on one end of the carbon chain in each alcohol molecule
(4) the location of an -OH group in the middle of the carbon chain in each alcohol molecule
3
49 What can be concluded from this graph?
(1) At 101.3 kPa, water has a higher boiling point than 1-butanol.
(2) At 101.3 kPa, water has a lower boiling point than ethanol.
(3) The greater the number of carbon atoms per alcohol molecule, the lower the boiling point of the alcohol.
(4) The greater the number of carbon atoms per alcohol molecule, the higher the boiling point of the alcohol.
50 In the laboratory, a student investigates the effect of concentration on the reaction between HCl(aq) and Mg(s), changing only the concentration of HCl(aq).
Data for two trials in the investigation are shown in the table below.
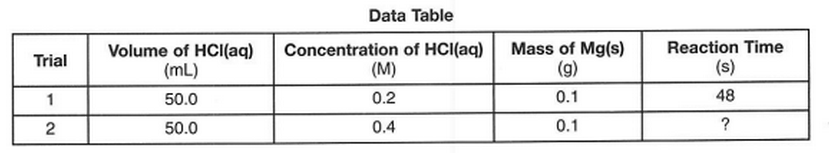
Compared to trial 1, what is the expected reaction time for trial 2 and the explanation for that result
(1) less than 48s, because there are fewer effective particle collisions per second
(2) less than 48s, because there are more effective particle collisions per second
(3) more than 48s, because there are fewer effective particle collisions per second
(4) more than 48s, because there are more effective particle collisions per second