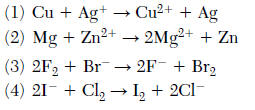Questions
Explanations
31 Which list of elements is arranged in order of increasing electronegativity?
(1) Be, Mg, Ca (3) K, Ca, Sc
(2) F, Cl, Br (4) Li, Na, K
32 The table below gives the masses of two different subatomic particles found in an atom.
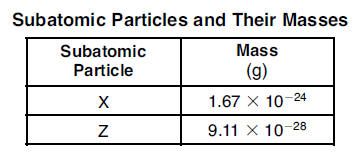
Which of the subatomic particles are each paired with their corresponding name?
(1) X, proton and Z, electron
(2) X, proton and Z, neutron
(3) X, neutron and Z, proton
(4) X, electron and Z, proton
33 Which electron configuration represents an excited state for an atom of calcium?
(1) 2-8-7-1 (3) 2-8-7-3
(2) 2-8-7-2 (4) 2-8-8-2
34 At STP, graphite and diamond are two solid forms of carbon. Which statement explains why these two forms of carbon differ in hardness?
(1) Graphite and diamond have different ionic radii.
(2) Graphite and diamond have different molecular structures.
(3) Graphite is a metal, but diamond is a nonmetal.
(4) Graphite is a good conductor of electricity, but diamond is a poor conductor of electricity.
35 Which equation shows conservation of charge?