Questions
Explanations
41 What is the total amount of heat required to completely melt 347 grams of ice at its melting point?
(1) 334 J (3) 116 000 J
(2) 1450 J (4) 784 000 J
42 As the temperature of a reaction increases, it is expected that the reacting particles collide
(1) more often and with greater force
(2) more often and with less force
(3) less often and with greater force
(4) less often and with less force43 Given the formula representing a compound:
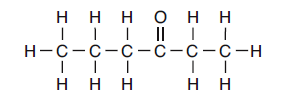
What is an IUPAC name for this compound?
(1) ethyl propanoate (3) 3-hexanone
(2) propyl ethanoate (4) 4-hexanone
44 A voltaic cell converts chemical energy to
(1) electrical energy with an external power source
(2) nuclear energy with an external power source
(3) electrical energy without an external power source
(4) nuclear energy without an external power source
45 Which acid and base react to form water and sodium sulfate?
(1) sulfuric acid and sodium hydroxide
(2) sulfuric acid and potassium hydroxide
(3) sulfurous acid and sodium hydroxide
(4) sulfurous acid and potassium hydroxide