Questions
Explanations
36 What occurs when potassium reacts with chlorine to form potassium chloride?
(1) Electrons are shared and the bonding is ionic.
(2) Electrons are shared and the bonding is covalent.
(3) Electrons are transferred and the bonding is ionic.
(4) Electrons are transferred and the bonding is covalent.
37 Given the balanced equation representing a reaction:
![]()
What occurs as bonds are broken in one mole of H2 molecules during this reaction?
(1) Energy is absorbed and one mole of unbonded hydrogen atoms is produced.
(2) Energy is absorbed and two moles of unbonded hydrogen atoms are produced.
(3) Energy is released and one mole of unbonded hydrogen atoms is produced.
(4) Energy is released and two moles of unbonded hydrogen atoms are produced.
38 Which pair of atoms has the most polar bond?
(1) H−Br (3) I−Br
(2) H−Cl (4) I−Cl
40 The graph below shows the volume and the mass of four different substances at STP. Which of the four substances has the lowest density?
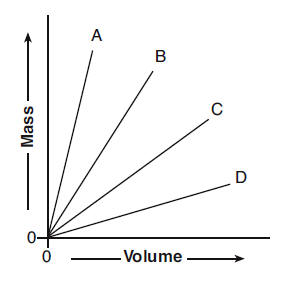
(1) A (3) C
(2) B (4) D