Questions
Explanations
46 Given the equation representing a reaction:
H2CO3 + NH3 → NH4+ + HCO3-
According to one acid-base theory, the compound NH3 acts as a base because it
(1) accepts a hydrogen ion
(2) donates a hydrogen ion
(3) accepts a hydroxide ion
(4) donates a hydroxide ion
47 Which statement describes characteristics of a 0.01 M KOH(aq) solution?
(1) The solution is acidic with a pH less than 7.
(2) The solution is acidic with a pH greater than 7.
(3) The solution is basic with a pH less than 7.
(4) The solution is basic with a pH greater than 7.
48 Four statements about the development of the atomic model are shown below.
A: Electrons have wavelike properties.
B: Atoms have small, negatively charged particles.
C: The center of an atom is a small, dense nucleus.
D: Atoms are hard, indivisible spheres.
Which order of statements represents the historical development of the atomic model?
(1) C → D → A → B
(2) C → D → B → A
(3) D → B → A → C
(4) D → B → C → A
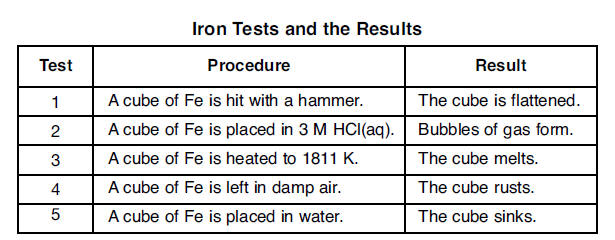
49 Five cubes of iron are tested in a laboratory. The tests and the results are shown in the table below.
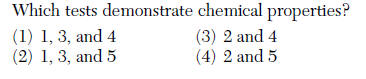
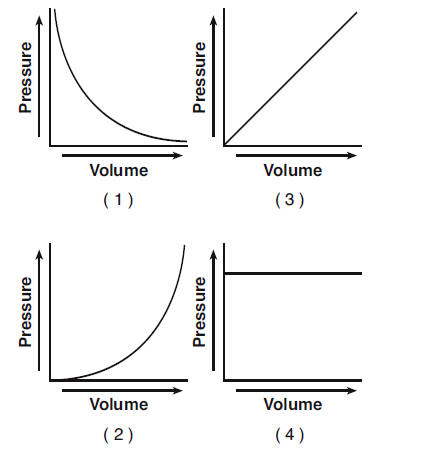
50 A rigid cylinder with a movable piston contains a sample of helium gas. The temperature of the gas is held constant as the piston is pulled outward. Which graph represents the relationship between the volume of the gas and the pressure of the gas?