|
Questions | Answer | Links | Explanations |
| 31 What is the number of electrons in an Al3+ ion? (1) 10 (3) 3 (2) 13 (4) 16 | 1 | Link | Metal ions have lost electrons #electrons = #protons-3 |
| 32 The valence electron of which atom in the ground state has the greatest amount of energy? (1) cesium (3) rubidium (2) lithium (4) sodium | 1 | Link | look for the atom with electrons in the highest energy level or look at the ionization energy |
| 33 The numbers of protons and neutrons in each of four different atoms are shown in the table below. 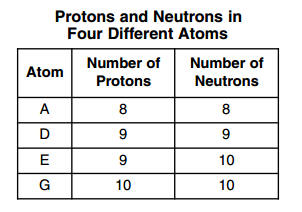
Which two atoms represent isotopes of the same element? (1) A and D (3) E and D (2) A and G (4) E and G | 3 | Link | Isotope-Same number of protons different number of neutrons |
|
| 34 Which elements have the most similar chemical properties? (1) boron and carbon (2) oxygen and sulfur (3) aluminum and bromine (4) argon and silicon | 2 | Link | Elements in the same group (above or below each other on the PT) |
| 35 Which element reacts with oxygen to form ionic bonds? (1) calcium (3) chlorine (2) hydrogen (4) nitrogen | 1 | Link | Metal and nonmetals=IONIC Bond look for a metal |
|
