Questions
Explanations
(1) chlorine (3) silver
(2) iodine (4) sulfur
(1) the same molecular structures and the same properties
(2) the same molecular structures and different properties
(3) different molecular structures and the same properties
(4) different molecular structures and different properties
(1) the atomic mass in grams
(2) the atomic number in grams
(3) the mass of neutrons in grams
(4) the number of neutrons in grams
CH4 + 2O2 → CO2 + 2H2O
According to this equation, what is the mole ratio of oxygen to methane?
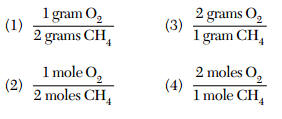
(1) decomposition, single replacement, and solidification
(2) decomposition, single replacement, and double replacement
(3) solidification, double replacement, and decomposition
(4) solidification, double replacement, and single replacement