Questions
Explanations
(1) KOH and CH3COOH
(2) KOH and C5H12
(3) CH3OH and CH3COOH
(4) CH3OH and C5H12
(1) Carbon and oxygen are both nonmetals.
(2) Carbon and oxygen have different electronegativities.
(3) The molecule has a symmetrical distribution of charge.
(4) The molecule has an asymmetrical distribution of charge.
(1) 15°C to 298 K (3) 305 K to 0°C
(2) 37°C to 273 K (4) 355 K to 25°C
C=K + 273
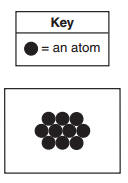
Which substance at STP can be represented by this particle diagram? (1) N2 (3) Mg
(2) H2 (4) Kr
(1) static phase equilibrium
(2) static solution equilibrium
(3) dynamic phase equilibrium
(4) dynamic solution equilibrium