|
Questions | Answer | Links | Explanations |
| 46 What are the products when potassium hydroxide reacts with hydrochloric acid? (1) KH(s), Cl-(aq), and OH-(aq) (2) K(s), Cl2(g), and H2O(ℓ) (3) KCl(aq) and H2O(ℓ) (4) KOH(aq) and Cl2(g) | 3 | Link | HCl(aq) + KOH(aq) → HOH(ℓ) + KCl(aq) |
| 47 In a titration, 20.0 milliliters of a 0.150 M NaOH(aq) solution exactly neutralizes 24.0 milliliters of an HCl(aq) solution. What is the concentration of the HCl(aq) solution? (1) 0.125 M (3) 0.250 M (2) 0.180 M (4) 0.360 M | 1 | Link | MV=MV M(24mL)=0.150M(20.0mL) |
| 48 What fraction of a Sr-90 sample remains unchanged after 87.3 years? 
| 4 | Link | Table N 87.1/29.1y= 3 half lives 1-->1/2-->1/4-->1/8 |
|
49 Which potential energy diagram represents the change in potential energy that occurs when a catalyst is added to a chemical reaction?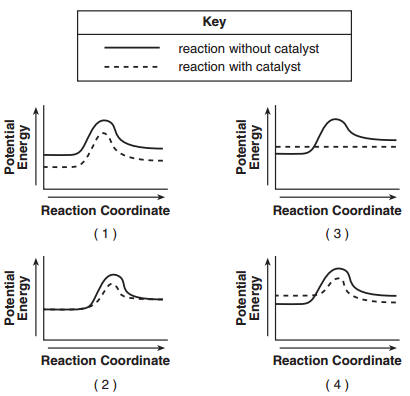 | 2 | Link | the hill becomes a bump. the PE of the reactants and products will not change |
| 50 Which balanced equation represents a spontaneous radioactive decay? 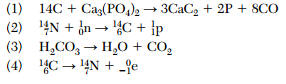
| 4 | Link | look for 1 reactant and a new element formed |
On to Question 51-53 |