Questions
Explanations
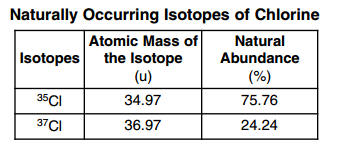
Which numerical setup can be used to calculate the atomic mass of the element chlorine?
(1) (34.97 u)(75.76) +(36.97 u)(24.24)
(2) (34.97 u)(0.2424) + (36.97 u)(0.7576)
(3) (34.97 u)(0.7576) + (36.97 u)(0.2424)
(4) (34.97 u)(24.24) + (36.97 u)(75.76) 37
(mass x % as a decimal) +
(mass x % as a decimal)
(1) The first ionization energy decreases and the electronegativity decreases.
(2) The first ionization energy increases and the electronegativity increases.
(3) The first ionization energy decreases and the electronegativity increases.
(4) The first ionization energy increases and the electronegativity decreases.
(1) 1 (3) 3
(2) 2 (4) 4
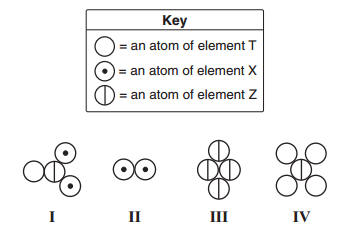
Which two models can be classified as elements?
(1) I and II (3) II and III
(2) I and IV (4) II and IV
(1) 25.0 g of KCl and 100. g of H2O
(2) 25.0 g of KNO3 and 100. g of H2O
(3) 25.0 g of NaCl and 100. g of H2O
(4) 25.0 g of NaNO3 and 100. g of H2O