Questions
Base your answers to questions 52 through 54 on the information below.
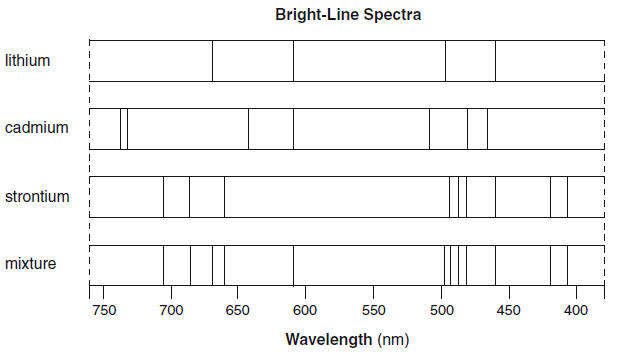
| The bright-line spectra for three elements and a mixture of elements are shown below.
52. Explain, in terms of both electrons and energy, how the bright-line spectrum of an element is produced. [1] HIGHLIGHT TO SEE THE ANSWER
53. Identify all the elements in the mixture. [1] HIGHLIGHT TO SEE THE ANSWER
54. State the total number of valence electrons in a cadmium atom in the ground state. [1] HIGHLIGHT TO SEE THE ANSWER
|
on to Questions 55-59