Questions
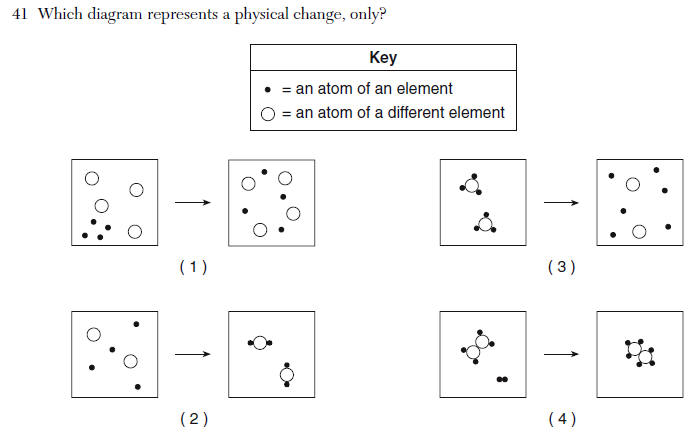
no bonds are formed, no new molecules are made
Reactions of Zn(s) with HCl(aq) | |||
| Experiment | Decription of Zinc Sample | HCl(aq) Concnetration(M) | Temperature (K) |
| 1 | lumps | 0.10 | 270. |
| 2 | powder | 0.10 | 270. |
| 3 | lumps | 0.10 | 290. |
| 4 | lumps | 1.0 | 290. |
| 5 | powder | 1.0 | 280. |
Which two experiements can be used to investigate the effect of the concentration of HCl(aq) on the reaction rate?
(1) 1 and 3 (2) 1 and 5 (3) 4 and 2 (4) 4 and 3
(1) from 400.K to 200.K
(2) from 200.K to 400.K
(3) from 400.oC to 200.oC
(4) from 200.oC to 400.oC
to double the volume the kelvin temperature must be doubled
(1) 8.0oC (2) 14oC (3) 30.oC (4) 55oC
q=mCDT
DT=q/mC=1200J/(36g x 4.18J/gC)= 8oC
22 + 8=30oC
(1) Molecules of Br2 are polar, and molecules of I2 are nonpolar.
(2) Molecules of I2 are polar, and molecules of Br2 are nonpolar.
(3) Molecules of Br2 have stronger intermolecular forces than molecules of I2.
(4) Molecules of I2 have stronger intermolecular forces than molecules of Br2.
(1) Ba(NO3)2 + Na2SO4 ==> BaSO4 + 2NaNO3
(2) H3PO4 + 3KOH==> K3PO4 + 3H2O
(3) Fe(s) + S(s) ==> FeS(s)
(4) NH3(aq) + HCl(aq)==> NH4Cl(s)
look for a free element (ox #=O) and that element in a compound (ox # not 0) on the other side
(1) KCl(aq) (2) CaO(aq) (3)NaOH(aq) (4)H2SO4(aq)
Acid start with Hydrogen
(1) 6.0mL (2) 12mL (3) 24mL (4) 48mL
2.0M(V)=1.0M(24mL)
V=12mL
(1) combustion (2) decomposition (3) nuclear fusion (4) oxidation-reduction
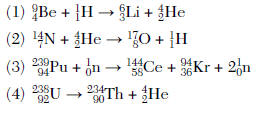
artificial has 2
transmutation is when 1 element becomes another by changing the number of protons