Polonium-210 occurs naturally, but is scarce. Polonium-210 is primarily used in devices designed to eliminate static electricity in machinery. It is also used in brushes to removedust from camera lenses.
Polonium-210 can be created in the laboratory by bombarding bismuth-209 with neutrons to create bismuth-210. The bismuth-210 undergoes beta decay to produce polonium-210. Polonium-210 has a half-life of 138 days and undergoes alpha decay.
83. State one beneficial use of Po-210. [1]
HIGHLIGHT TO SEE THE ANSWER
from article- Po-210 is used to eliminate static electricity in machinery or removes dust from camera lenses |
84. Complete the nuclear equation in your answer booklet for the decay of Po-210, by writing a notation for the missing product. [1]
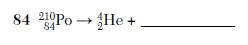
HIGHLIGHT TO SEE THE ANSWER
85. Determine the total mass of an original 28.0-milligram sample of Po-210 that remains unchanged after 414 days. [1]
HIGHLIGHT TO SEE THE ANSWER
Answer =3.5mg # of halflive=414d/138=3 halflives 28.0mg==>14.0==>7.0==>3.5mg |