Questions
Base your answers to questions 77 through 79 on the information below.
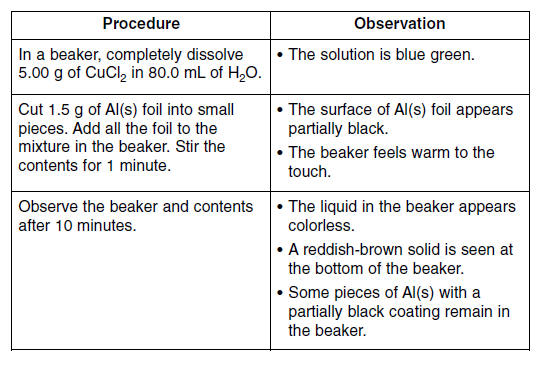
A student performed a laboratory activity to observe the reaction between aluminum foil and an aqueous copper(II) chloride solution. The reaction is represented by the balanced equation below. 2Al(s) + 3CuCl2(aq) → 3Cu(s) + 2AlCl3(aq) + energy The procedures and corresponding observations for the activities are given below.
77. State one observation that indicates Cu2+ ions became Cu atoms. [1] HIGHLIGHT TO SEE THE ANSWER
78. Describe one change in the procedure that would cause the reaction to occur at a faster rate. [1] HIGHLIGHT TO SEE THE ANSWER
79. State one safety procedure the student should perform after completing the laboratory activity. [1] HIGHLIGHT TO SEE THE ANSWER
|
on to Questions 80-82