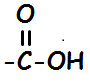Questions | Answer | Link | Explanations |
| 21 Given the formula of the functional group: 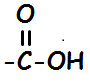
An organic compound that has this functional group is classified as (1) an acid (2) an aldehyde (3) an ester (4) a ketone | 1 | Link | this is an organic acid look at table R |
| 22 Which statement describes where oxidation and reduction half-reactions occur in an operating electrochemical cell? (1) Oxidation and reduction both occur at the anode (2) Oxidation and reduction both occur at the cathode (3) Oxidation occurs at the anode and reduction occurs at the cathode (4) Oxidation occurs at the cathode and reduction occurs at the anode | 3 | Link | An OX Anode-oxidation Red Cat Cathode reduction |
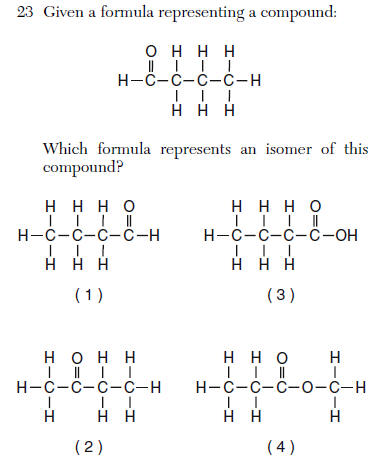 | 2 | Link | move the C=O to another carbon |
| 24. Which energy conversion occurs in an operating electrolytic cell? (1) chemical energy to electrical energy (2) electrical energy to chemical energy (3) nuclear energy to thermal energy (4) thermal energy to nuclear energy | 2 | Link | electrolytic requires a power source for a reaction to occur |
| 25 Which compounds are classified as electrolytes? (1) alcohols (2) alkynes (3) organic acids (4) saturated hydrocarbons | 3 | Link | electrolytes are acids bases and soluble salts (ionic) |
| 26 Potassium hydroxide is classified as an Arrhenius base because KOH contains (1) OH- ions (2) O2- ions (3) K+ ions (4) H+ ions | 1 | Link | Arrhenius bases contain OH- |
| 27 In which laboratory process is a volume of solution of known concentration used to determine the concentration of another solution? (1) deposition (2) distillation (3) filtration (4) titration | 4 | Link | titrations use a known volume and concentration (usually acids or bases) to determine the concentration of an unknown, using an indicator to determine the equivalence point |
| 28 According to one acid-base theory, an acid is an (1) H+ acceptor (2) H+ donor (3) OH- acceptor (4) OH- donor | 2 | Link | Bronsted Lowery (the other theory) acids are proton (H+) donors and bases accept protons |
| 29 Energy is released during the fission of Pu-239 atoms as a result of the (1) formation of covalent bonds (2) formation of ionic bonds (3) conversion of matter to energy (4) conversion of energy to matter | 3 | Link | Energy releases from fission is due to matter being converted to energy E=mc2 |
| 30 Atoms of I-131 spontaneously decay when the (1) stable nuclei emit alpha particles (2) stable nuclei emit beta particles (3) unstable nuclei emit alpha particles (4) unstable nuclei emit beta particles | 4 | Link | atoms decay because of unstable nuclei I-131 has a beta minus decay (Table N) |