Questions
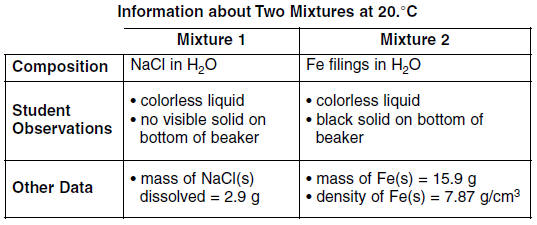
Base your answers to questions 74 through 76 on the information below.
A student prepared two mixtures, each in a labeled beaker. Enough water at 20.°C was used to make 100 milliliters of each mixture.
74. Classify each mixture using the term “homogeneous” or the term “heterogeneous.” [1] HIGHLIGHT TO SEE THE ANSWER
75. Determine the volume of the Fe filings used to produce mixture 2. [1] HIGHLIGHT TO SEE THE ANSWER
76. Describe a procedure to physically remove the water from mixture 1. [1] HIGHLIGHT TO SEE THE ANSWER
|
on to Questions 77-79