Questions
Base your answers to questions 62 through 63 on the information below.
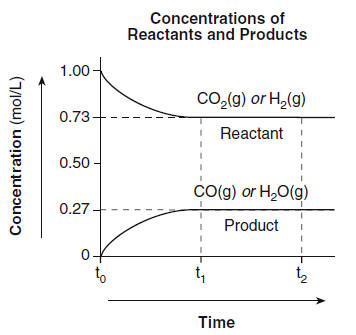
At 550°C, 1.00 mole of CO2(g) and 1.00 mole of H2(g) are placed in a 1.00-liter reaction vessel. The substances react to form CO(g) and H2O(g). Changes in the concentrations of the reactants and the concentrations of the products are shown in the graph below.
62. Determine the change in the concentration of CO2(g) between time t0 and time t1. [1] HIGHLIGHT TO SEE THE ANSWER
63. What can be concluded from the graph about the concentrations of the reactants and the concentrations of the products between time t1 and time t2? [1] HIGHLIGHT TO SEE THE ANSWER
|
on to Questions 64-65