Questions
Base your answers to questions 69 through 70 on the information below.
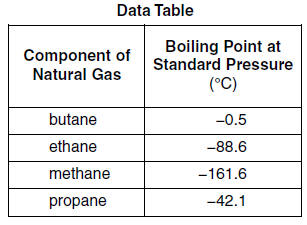
Natural gas is a mixture that includes butane, ethane, methane, and propane. Differences in boiling points can be used to separate the components of natural gas. The boiling points at standard pressure for these components are listed in the table below.
69. Identify a process used to separate the components of natural gas. [1] HIGHLIGHT TO SEE THE ANSWER
70. List the four components of natural gas in order of increasing strength of intermolecular forces. [1] HIGHLIGHT TO SEE THE ANSWER
|
on to Questions 71-73